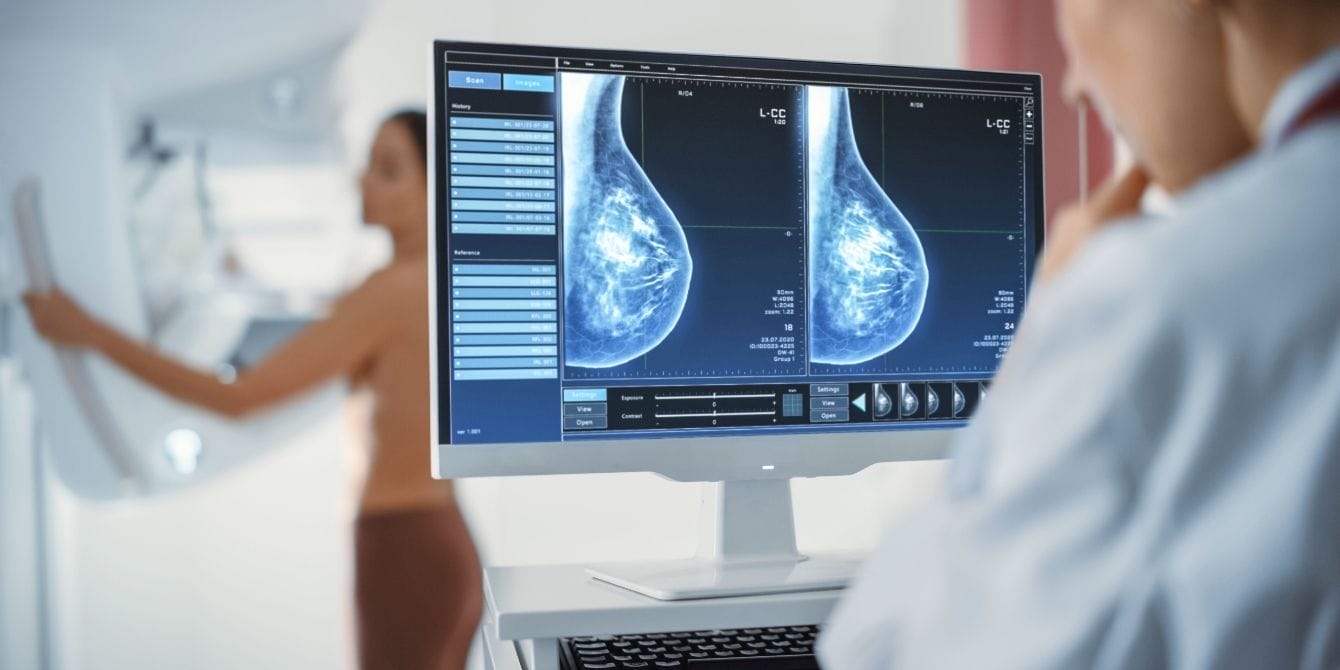
Many women don’t know that having dense breast tissue can put them at higher risk for breast cancer — and make cancer harder to spot and diagnose. But how do you even know if you have dense breast tissue? Starting this week, all mammograms in the U.S. will be required to include assessment reports on breast density, according to a new FDA rule that went into effect Tuesday.
Breast density is a measurement of how much fibroglandular tissue a person has in their breast compared to fatty tissue. According to radiologist Dr. Kimberly Feigin, interim chief of the Breast Imaging Service and head of the Breast Imaging Quality Assurance at Memorial Sloan Kettering Cancer Center, about half of women over 40 in the U.S. have dense breasts.
“We talk about breast density for two reasons. One is that breast density can make it more difficult to spot a cancer on a mammogram, because dense breast tissue – the glandular elements and connective tissue supporting elements – looks white on a mammogram and cancer also looks white on a mammogram,” told CNN. “The second reason that breast density is important is because having dense breast tissue raises a woman’s level of risk of developing breast cancer.”
The new FDA rule doesn’t provide specific next steps for people who have dense breast tissue. But the idea is that those who do can talk to their doctors to better understand the risks and develop a screening plan that’s right for them. That may include getting MRIs or ultrasounds, which can sometimes better detect breast cancer in dense tissue.
Breast cancer survivor JoAnn Pushkin, 64, has been advocating for more than 10 years for better rules around breast density disclosure. Around 20 years ago, Pushkin found a lump in her breast. Since she had had a mammogram that appeared normal just eight weeks earlier, she wasn’t worried. But still, she scheduled a diagnostic mammogram, where the radiologists said they couldn’t see anything.
“It was a large facility with multiple waiting rooms, and I just assumed that she’d come back into the wrong room,” Pushkin said. “I said, ‘Oh no, I’m the lady with the lump so big I could feel it.’ And she told me, ‘Oh, you have dense breasts. That’s going to be a very hard find for us.’ And I remember sitting back and saying to her, ‘Wait, what?’ I didn’t even know how to make sense of that sentence.”
Pushkin decided to pursue further testing, so she had an ultrasound performed.
“And there was the lump, clear as a bell,” she said. “It was determined to be breast cancer. Within 20 minutes, I learned I had dense breasts, I learned I had breast cancer, and I learned it had been missed because I had dense breasts.”
Because Pushkin was diagnosed at a later stage, her cancer required eight surgeries and eight rounds of chemotherapy. She later had a recurrence and underwent radiation.
The new FDA rule may help more people become informed about the challenges of diagnosing breast cancer in dense breast tissue, and avoid stories like Joan’s.
“I feel that because I wasn’t told I have dense breasts, I was effectively denied the opportunity for an early-stage diagnosis,” she said.